adagrasib (MRTX849), an oral KRAS G12C inhibitor developed by Mirati Therapeutics, is on the market
2022-12-15
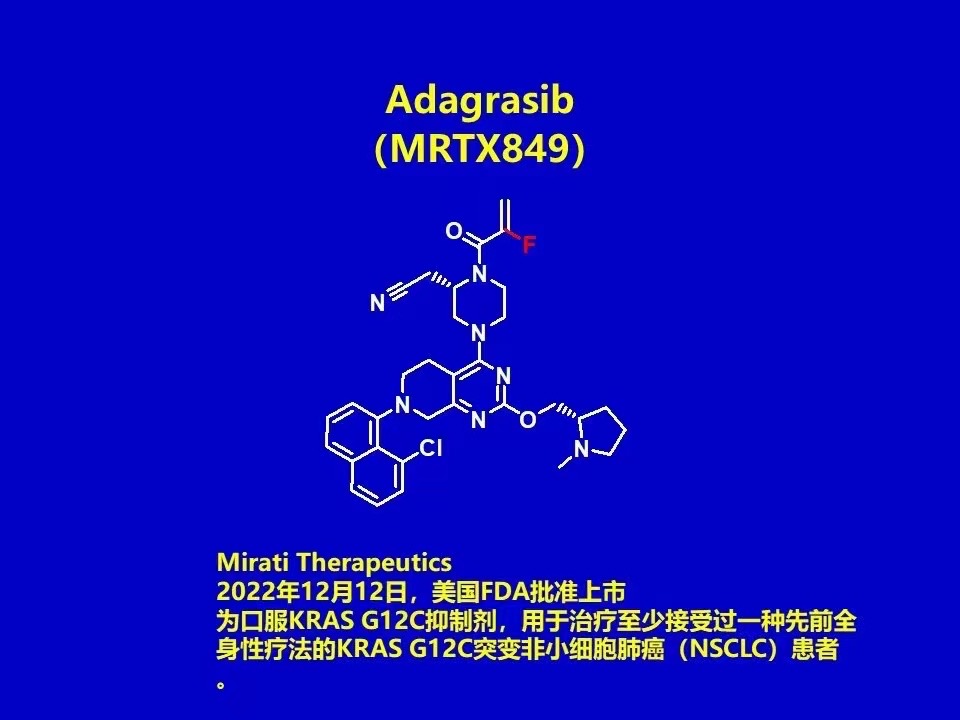
2022.12.12, the US FDA announced the approval of Mirati Therapeutics' oral KRAS G12C inhibitor adagrasib (MRTX849), To treat patients with KRAS G12C mutated non-small cell lung cancer (NSCLC) who have received at least one prior systemic therapy. It is the second KRAS G12C inhibitor to be marketed globally after Lumakras (sotorasib). In June 2021, the product was granted breakthrough therapy designation by the FDA. Amgen's sotorasib, the first KRAS G12C inhibitor marketed globally, was approved in May 2021 for the second-line treatment of patients with advanced or metastatic NSCLC with KRAS G12C mutations.
